Sysmex SE 9500
| Objektnummer | B00009656 |
|---|---|
| Seriennummer | 009656 |
| Object Naam | Sysmex SE 9500 |
| Status | Stock unit |
Product groep: Hematologie
Status, leverings- en betalingsvoorwaarden
Apparatuurcontrole
De gebruikte apparatuur wordt voorafgaand aan levering gecontroleerd door Labexchange Service GmbH. U ontvangt volledig functionerende apparatuur.
Verzending
De vermelde verzendtijden zijn telkens de kortste voor een artikel. In bepaalde gevallen kunnen de daadwerkelijke verzendtijden daarvan afwijken. De uiteindelijke verzendtijden worden aangegeven in de opdrachtbevestiging.
In de regel bieden we combinatieleveringen aan. Levertijden zijn afhankelijk van het artikel met de langste levertijd. Deelleveringen zijn mogelijk tegen een toeslag.
Verzendmethoden
Koeriersdiensten, transportbedrijven, zelf afhalen, levering door Labexchange wagenpark
Informatie levering
De prijzen zijn exclusief verzendkosten. De genoemde verzendkosten zijn de te verwachten kosten. Afwijkingen zijn mogelijk. In het geval geen kosten voor verzending zijn gespecificeerd, vraag die dan afzonderlijk aan.
De opgegeven vracht- en verpakkingskosten hebben betrekking op de goedkoopste transportroute en zijn onder voorbehoud van onvoorziene kostenstijgingen. Door onvoorziene gebeurtenissen kunnen vrachttarieven en levertijden op elk moment veranderen en moeten ze worden aangepast aan de huidige situatie. Incoterm coderingen volgens Incoterms 2010: Bij afhalen EXW, CFR voor zendingen over zee, CPT per luchtfracht, andere zendingen DAP. Opmerking: We geven geen preferentieel certificaat/EUR1 af. Bij zelf afhalen/af fabriek (EXW) uit derde landen en de EU wordt 16% btw als borg ingehouden, tot we de ontvangstbevestiging/het leveringscertificaat van de koper hebben ontvangen.
Betalingsvoorwaarden
Wij accepteren geen betalingen Letter of Credit, PayPal etc. Het factuurbedrag is volledig verschuldigd. Er zijn geen betalingskortingen. De goederen blijven tot volledige betaling ons eigendom.
|
Land |
Mogelijke betaalmethoden |
Opmerking |
|
Duitsland, Oostenrijk, Zwitserland |
Betaling via factuur, vooruitbetaling, per creditkaart |
Betaling via factuur is mogelijk voor ondernemingsklanten. |
|
Nederland, België en Luxemburg |
Betaling via factuur, vooruitbetaling, per creditkaart |
Betaling via factuur is mogelijk voor ondernemingsklanten. |
|
Andere landen |
vooruitbetaling, per creditkaart |
|
Onze Algemene Voorwaarden voor Verkoop, Levering en Betaling zijn hierop van toepassing. Deze voorwaarden zijn hier te downloaden.
Tussenverkoop is ons voorbehouden.
Beschrijving status:
Alle artikelen zijn gebruikte artikelen, tenzij bij een artikel uitdrukkelijk wordt vermeld dat het om een nieuw apparaat gaat.
Kommentar: Dokumente engl. (für ID. 13860)
The following illustrations and descriptions are referring to the instrument model and are drawn from brochures. They are not representing the delivery volume. The exact delivery content you will find only in the offering text.
INTRODUCTION
The Sysmex© SE-9500 is a technically advanced, computerized, fully automated hematology analyzer with many features, for in vitro diagnostic use in clinical laboratories. The SE-9500 provides accurate screening for patients whose hematologic abnormalities indicate the need for further testing. In addition, it can contribute to diagnosis, patient monitoring and judgment of treatment effectiveness.
The SE-9500 has the capability of analyzing up to 120 samples per hour, and displays 6 different types of data patterns -- WBC 3 differential, immature cell scattergrams, and cell size distribution curves for eosinophils, basophils, red blood cells and thrombocytes, as well as 23 analysis data parameters on the DMS (data management system) screen.
Chapter 1 is an introduction to this manual, major system components, system operation and other general considerations. This chapter should be read before operating the SE-9500. Chapter 1 contains the following subjects:
Manual Organization
A description of the Operator's Manual and explanation of important informational headings.
System Overview
An introduction to the main elements of the SE-9500 analyzer, including a description of the options available to enhance the instrument's operation.
Safety Summary
Covers major safety considerations when using and servicing the SE-9500.
Operational Summary
A summary of the operating procedures for each mode, the DMS screen display, names and functions of keyboard keys, etc.
Installation
Includes space requirements, necessary equipment, installation environment and other essential information to be reviewed before installation of the SE-9500.
Instrument Specifications
The instrument's specifications are given in a reference table.
SYSTEM OVERVIEW
The purpose of the SE-9500 is to provide accurate results an 23 parameters and to detect samples that present an abnormality. To assist the laboratory in the screening of abnormals, the SE-9500 analyzes and processes the data obtained from the analyzer unit, and the DMS displays and prints either a POSITIVE or a NEGATIVE message for each sample. A NEGATIVE message signifies that parameter values for the sample are within acceptable limits and the sample does not require any additional verification of those values. A POSITIVE message signifies that the sample is not within the acceptable limits and requires further review and investigation. Proper use of the instrument requires laboratory to establish the acceptable limits for results.
The SE-9500 uses four detector blocks and four types of reagent to analyze the WBC populations. In the DIFF detector block, lymphocyte, granulocyte and monocyte cells are treated with diluent and lyse reagents are analyzed using the Radio Frequency (RF) and Direct Current (DC) detection methods. The WBC detector block uses a mixture of diluent and lyse reagents and the DC detection method to determine the number of WBCs. Eosinophil and Basophil determinations are performed by the EO and BASO detector blocks respectively, using the DC detection method and a cell-specific lysing reagents. RBCs and PLTs are analyzed by the RBC detector block using the sheath-flow DC detection method. HGB determination is performed using the SLS hemoglobin (or Cyanmethemoglobin) detection method. Also, the immature cell information is determined qualitatively by the IMI detector block using the RFDC detection method.
The SE-9500 consists of five major systems.
Main Unit Houses the hydraulic and electronic system for analysis and their control components.
Sampler Unit Consists of a mixer, cap piercer unit which supplies samples to the Main Unit.
DMS Processes and displays data obtained from the Main Unit.
Pneumatic Unit Supplies the required pressure and vacuum for analysis.
Power Supply Unit Supplies power to the Main Unit.
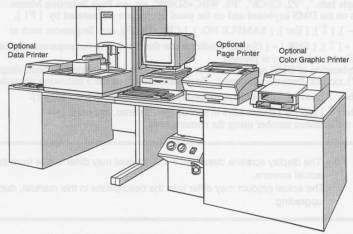
Sysmex SE-9500 Hematology Analyzer
System Specifications
Parameters WBC, NEUT%, LYMPH%, MONO%, EO%, BASO%, NEUT#, LYMPH#, MONO#, EO#, BASO#, RBC, HGB, HCT, MCV, MCH, MCHC, RDW-SD, RDW-CV, PLT, PDW, MPV, P- LCR
Throughput Approximately 120 samples per hour
(CBC + Diff: Approx. 120 seconds per sample. CBC: Approx. 70 seconds per sample)
Principles RF/DC Detection Method (MONO, GRAN, IMI)
DC Detection Method (WBC, LYMPH, EO, BASO)
Sheath-Flow DC Detection Method (RBC, PLT)
SLS Hemoglobin Method or Cyanmethemoglobin Method (HGB)
Cumulative Pulse Height Detection Method (HCT)
MCV (Calculated from RBC and HCT)
MCH (Calculated from RBC and HGB)
MCHC (Calculated from HCT and HGB)
NEUT (Calculated from WBC, EO, BASO, LYMPH, MONO)
Sample Volume Required Sampler Mode Approximately 250 µL
Closed Mode Approximately 250 µL
Manual Mode Approximately 100 µL
Capillary Mode Over 40 µL
Data Storage Capacity: Cell size distribution data (with graphs): for 10,000 samples
Scattergrams for 10,000 samples
Patient information for 5,000 patients
Order information for 1,000 samples
Dimensions Main Unit & Sampler Unit Approx. 720 x 636 x 820
H x W x D (mm) Power Supply Unit Approx. 215 x 285 x 325
Pneumatic Unit Approx. 390 x 340 x 500
Data Management System (DMS) Approx. 486 x 472 x 618
Power Requirement 117 VAC ± 10%, 220 VAC ± 10%, 240 VAC ± 10% (50 or 60 Hz)
Power Consumption Power Supply Unit (Main & Sampler Unit):
117 VAC, 50 Hz (or 60 Hz) 820 VA
220 VAC, 50 Hz (or 60 Hz) 870 VA
240 VAC, 50 Hz (or 60 Hz) 870 VA
Pneumatic Unit:
400 VA, 50 Hz (or 60 Hz)
Data Management System: 600 VA, 50 Hz (or 60 Hz)
Temperature Approx. 1196 kcal/h (4743 BTU/h)
Compensation Required (Excluding DMS and printers)
Protection Type I Class I equipment
RAM 1
Accurate
► more than 30.00 RBC's per cycle are analyzed: highest precision even at low reticulocyte ► concentrations allows monitroing of patient treatment and therapy.
► Optimum light source for particle analysis by high power argon laser
► Golden Standard technology for reticulocyte analysis
► Fixed volume: absolute counting eliminates need for system calibration
► QC material with IQAS support (International Quality Assurance System)
Innovative parameter
► Platelet (O) as verification option for PLT-count accuracy in extremely pathological samples.
► IRF parameter for monitoring patient treatment
Easy to operate
► Fully automated with autosampler
► Sample identification by barcode
► Review support by download of patient demographics from HOST computer
► Rapid patient oriented validation with cumulative data review display
Principle & Technologies
Flow Cytometry with argon laser
Two detection wavelengths
Cetl RNA/DNA stain:
Auramine-0 (Fluorochrome)
Parameters
RET %, RET#, RBC, LFR, MFR, HFR
Research Mode: PLT(0)
Throughput
72 -119 samples/h
Sample Volume
100 µl (manual mode)
250 µl (auto mode)
40 µl (capillary mode)
Data Storage
10.000 samples, including scattergramns and histograms
Communication
Bi-directional
Quality Control
16 flies with 180 data-points
Interfaces
Serial: HOSE Computer, Line Printer
Parallel: Data- and Graphic Printer
Dimensions (WxDxH, mm)
Weights (kg) Main Unit 400x505X720/46
Laser Unit 222X290X216/23
Power Supply Unit 221x285x148/8,7
Pneumatic Unit 222x290x216/16,5
Configurations
Requires operation connected
with AMS main unit (SE-9000/9500)







