Sysmex SE 9500
| Objektnummer | B00009656 |
|---|---|
| Seriennummer | 009656 |
| Nome oggetto | Sysmex SE 9500 |
| Stato | Archived Product |
Gruppo prodotti: Ematologia
Status, terms of delivery and payment
Verification of devices
The second-hand devices are verified by Labexchange Service GmbH before delivery. You are receiving only fully functional devices.
Dispatch time
The stated dispatch times are the shortest possible ones for each article. The effective dispatch times can vary. The effective dispatch times will be stated in the order confirmation.
As a matter of principle, we are offering collective deliveries. The shipping time is calculated based on the position with the longest lead time. A partial delivery is possible on explicit request.
Shipping methods
Parcel services, forwarding agencies, self-pickup, delivery by Labexchange fleet.
Delivery information
Prices exclude shipping costs. Stated shipping costs are to be expected. Deviations are possible. If transport costs are not specified, please ask separately for them.
The stated transport and packing charges apply to the most favorable route if transport and are to be understood as subject to verification due to unexpected cost increases. By reason of unpredictable events, cargo rates and delivery times can change at any time and therefore have to be adapted to the recent situation. Import formalities and possible customs charges will be borne by the purchaser. Incoterm coding according to Incoterms 2010: For persons who collect the devices themselves: EXW, for dipatch by sea: CFR, by air freight: CPT, other shipments: DAP. Note for international shipments: A proof of preference/EUR1 will not be issued by us. When self-collecting/ordering EXW from countries within or outside the European Union, 16% VAT will be retained as a deposit until we have received the corresponding confirmation of arrival/bill of delivery from the buyer.
Terms of payment
We do not accept payment by letter of credit, PayPal, etc. In each case the invoice amount is payable without deduction. Discount is not granted.
|
Country |
Possible payment methods |
Comment |
|
DE, AT, CH |
Payment by invoice, payment in advance, payment by credit card |
Payment by invoice is only possible for corporate clients. |
|
NL, BE, LU |
Payment by invoice, payment in advance, payment by credit card |
Payment by invoice is only possible for corporate clients |
|
Other countries |
Payment in advance, payment by credit card |
|
Our General Terms of Sale, Delivery and Payment are valid and are available for download here.
The goods are offered subject to prior sale.
Definition of status
All articles are used articles, except an article is listed especially as a new device.
|
Status |
Condition |
Comment |
|
Immediately available |
Used | The article is fully functional and in impeccable condition. It can be shipped immediately. |
| In stock |
Used |
The article is on stock. Our service technicians will verify the article before delivery. You are receiving only a fully functional article. |
|
Published |
Used |
The article is still with the provider. After your order the article will be purchased and verified by us before being shipped to you. A certificate of operativeness as well as a service report are included in delivery. |
|
New device |
new |
The article is brand new and unused. Regarding new equipment the guarantee/warranty conditions of the corresponding manufacturer apply. |
|
Labprocure |
Used |
Labprocure GmbH, as the advertiser, is responsible for the content of this device offer. Labprocure assumes liability for the offers advertised here and for the photos and offer texts included. Labprocure GmbH, Bruckstraße 58, 72393 Burladingen. |
Kommentar: Dokumente engl. (für ID. 13860)
The following illustrations and descriptions are referring to the instrument model and are drawn from brochures. They are not representing the delivery volume. The exact delivery content you will find only in the offering text.
INTRODUCTION
The Sysmex© SE-9500 is a technically advanced, computerized, fully automated hematology analyzer with many features, for in vitro diagnostic use in clinical laboratories. The SE-9500 provides accurate screening for patients whose hematologic abnormalities indicate the need for further testing. In addition, it can contribute to diagnosis, patient monitoring and judgment of treatment effectiveness.
The SE-9500 has the capability of analyzing up to 120 samples per hour, and displays 6 different types of data patterns -- WBC 3 differential, immature cell scattergrams, and cell size distribution curves for eosinophils, basophils, red blood cells and thrombocytes, as well as 23 analysis data parameters on the DMS (data management system) screen.
Chapter 1 is an introduction to this manual, major system components, system operation and other general considerations. This chapter should be read before operating the SE-9500. Chapter 1 contains the following subjects:
Manual Organization
A description of the Operator's Manual and explanation of important informational headings.
System Overview
An introduction to the main elements of the SE-9500 analyzer, including a description of the options available to enhance the instrument's operation.
Safety Summary
Covers major safety considerations when using and servicing the SE-9500.
Operational Summary
A summary of the operating procedures for each mode, the DMS screen display, names and functions of keyboard keys, etc.
Installation
Includes space requirements, necessary equipment, installation environment and other essential information to be reviewed before installation of the SE-9500.
Instrument Specifications
The instrument's specifications are given in a reference table.
SYSTEM OVERVIEW
The purpose of the SE-9500 is to provide accurate results an 23 parameters and to detect samples that present an abnormality. To assist the laboratory in the screening of abnormals, the SE-9500 analyzes and processes the data obtained from the analyzer unit, and the DMS displays and prints either a POSITIVE or a NEGATIVE message for each sample. A NEGATIVE message signifies that parameter values for the sample are within acceptable limits and the sample does not require any additional verification of those values. A POSITIVE message signifies that the sample is not within the acceptable limits and requires further review and investigation. Proper use of the instrument requires laboratory to establish the acceptable limits for results.
The SE-9500 uses four detector blocks and four types of reagent to analyze the WBC populations. In the DIFF detector block, lymphocyte, granulocyte and monocyte cells are treated with diluent and lyse reagents are analyzed using the Radio Frequency (RF) and Direct Current (DC) detection methods. The WBC detector block uses a mixture of diluent and lyse reagents and the DC detection method to determine the number of WBCs. Eosinophil and Basophil determinations are performed by the EO and BASO detector blocks respectively, using the DC detection method and a cell-specific lysing reagents. RBCs and PLTs are analyzed by the RBC detector block using the sheath-flow DC detection method. HGB determination is performed using the SLS hemoglobin (or Cyanmethemoglobin) detection method. Also, the immature cell information is determined qualitatively by the IMI detector block using the RFDC detection method.
The SE-9500 consists of five major systems.
Main Unit Houses the hydraulic and electronic system for analysis and their control components.
Sampler Unit Consists of a mixer, cap piercer unit which supplies samples to the Main Unit.
DMS Processes and displays data obtained from the Main Unit.
Pneumatic Unit Supplies the required pressure and vacuum for analysis.
Power Supply Unit Supplies power to the Main Unit.
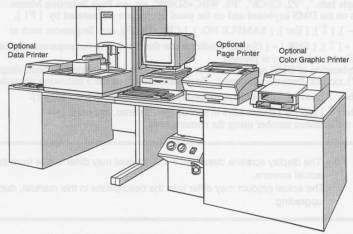
Sysmex SE-9500 Hematology Analyzer
System Specifications
Parameters WBC, NEUT%, LYMPH%, MONO%, EO%, BASO%, NEUT#, LYMPH#, MONO#, EO#, BASO#, RBC, HGB, HCT, MCV, MCH, MCHC, RDW-SD, RDW-CV, PLT, PDW, MPV, P- LCR
Throughput Approximately 120 samples per hour
(CBC + Diff: Approx. 120 seconds per sample. CBC: Approx. 70 seconds per sample)
Principles RF/DC Detection Method (MONO, GRAN, IMI)
DC Detection Method (WBC, LYMPH, EO, BASO)
Sheath-Flow DC Detection Method (RBC, PLT)
SLS Hemoglobin Method or Cyanmethemoglobin Method (HGB)
Cumulative Pulse Height Detection Method (HCT)
MCV (Calculated from RBC and HCT)
MCH (Calculated from RBC and HGB)
MCHC (Calculated from HCT and HGB)
NEUT (Calculated from WBC, EO, BASO, LYMPH, MONO)
Sample Volume Required Sampler Mode Approximately 250 µL
Closed Mode Approximately 250 µL
Manual Mode Approximately 100 µL
Capillary Mode Over 40 µL
Data Storage Capacity: Cell size distribution data (with graphs): for 10,000 samples
Scattergrams for 10,000 samples
Patient information for 5,000 patients
Order information for 1,000 samples
Dimensions Main Unit & Sampler Unit Approx. 720 x 636 x 820
H x W x D (mm) Power Supply Unit Approx. 215 x 285 x 325
Pneumatic Unit Approx. 390 x 340 x 500
Data Management System (DMS) Approx. 486 x 472 x 618
Power Requirement 117 VAC ± 10%, 220 VAC ± 10%, 240 VAC ± 10% (50 or 60 Hz)
Power Consumption Power Supply Unit (Main & Sampler Unit):
117 VAC, 50 Hz (or 60 Hz) 820 VA
220 VAC, 50 Hz (or 60 Hz) 870 VA
240 VAC, 50 Hz (or 60 Hz) 870 VA
Pneumatic Unit:
400 VA, 50 Hz (or 60 Hz)
Data Management System: 600 VA, 50 Hz (or 60 Hz)
Temperature Approx. 1196 kcal/h (4743 BTU/h)
Compensation Required (Excluding DMS and printers)
Protection Type I Class I equipment
RAM 1
Accurate
► more than 30.00 RBC's per cycle are analyzed: highest precision even at low reticulocyte ► concentrations allows monitroing of patient treatment and therapy.
► Optimum light source for particle analysis by high power argon laser
► Golden Standard technology for reticulocyte analysis
► Fixed volume: absolute counting eliminates need for system calibration
► QC material with IQAS support (International Quality Assurance System)
Innovative parameter
► Platelet (O) as verification option for PLT-count accuracy in extremely pathological samples.
► IRF parameter for monitoring patient treatment
Easy to operate
► Fully automated with autosampler
► Sample identification by barcode
► Review support by download of patient demographics from HOST computer
► Rapid patient oriented validation with cumulative data review display
Principle & Technologies
Flow Cytometry with argon laser
Two detection wavelengths
Cetl RNA/DNA stain:
Auramine-0 (Fluorochrome)
Parameters
RET %, RET#, RBC, LFR, MFR, HFR
Research Mode: PLT(0)
Throughput
72 -119 samples/h
Sample Volume
100 µl (manual mode)
250 µl (auto mode)
40 µl (capillary mode)
Data Storage
10.000 samples, including scattergramns and histograms
Communication
Bi-directional
Quality Control
16 flies with 180 data-points
Interfaces
Serial: HOSE Computer, Line Printer
Parallel: Data- and Graphic Printer
Dimensions (WxDxH, mm)
Weights (kg) Main Unit 400x505X720/46
Laser Unit 222X290X216/23
Power Supply Unit 221x285x148/8,7
Pneumatic Unit 222x290x216/16,5
Configurations
Requires operation connected
with AMS main unit (SE-9000/9500)







